Reports
Product Recall Report
The Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (Bioterrorism Act) and the Food Safety Modernization Act (FSMA), signed into law in 2011, are having and will continue to have an impact on the global food supply chain. Distributors must be prepared to meet the new regulations or face large fines and possibly closure. These laws give the Food and Drug Administration unprecedented new powers of enforcement, inspection, and forced recall. Because of this, it was especially important that we include lot tracking features that will keep our customers in adherence to this new law and the freedom of mind that they are prepared in case of a product recall.
The Electronic Warehouse Manager will help you with becoming compliant by using the Lot Number (or Production Date) encoded in the manufacturer's barcode label, without the need for you to enable "Lot Tracking" in your entrée software.
To put it simply, when inventory is received, the scanned lot number in the label is recorded (along with all other key information). When the product is sold, the lot number in the scanned label is recorded, including all details including the customer that purchased the item.
entrée Lot Number Assignment Warehouse staff cannot assign lots in EWM. You have 3 possible methods for getting Lot numbers assigned in the entrée system.
1.Your office staff can manually assign lot numbers in entrée during invoicing. 2.You can turn on entrée system option #106 to auto-assign lots during invoicing. 3.You can scan the lot number embedded in the barcode label that comes on the product during receiving.
Once one of the 3 options lot number assignments options above are implemented entrée can automatically assign the Lot Numbers and other key information in EWM to meet your labeling requirements.
|
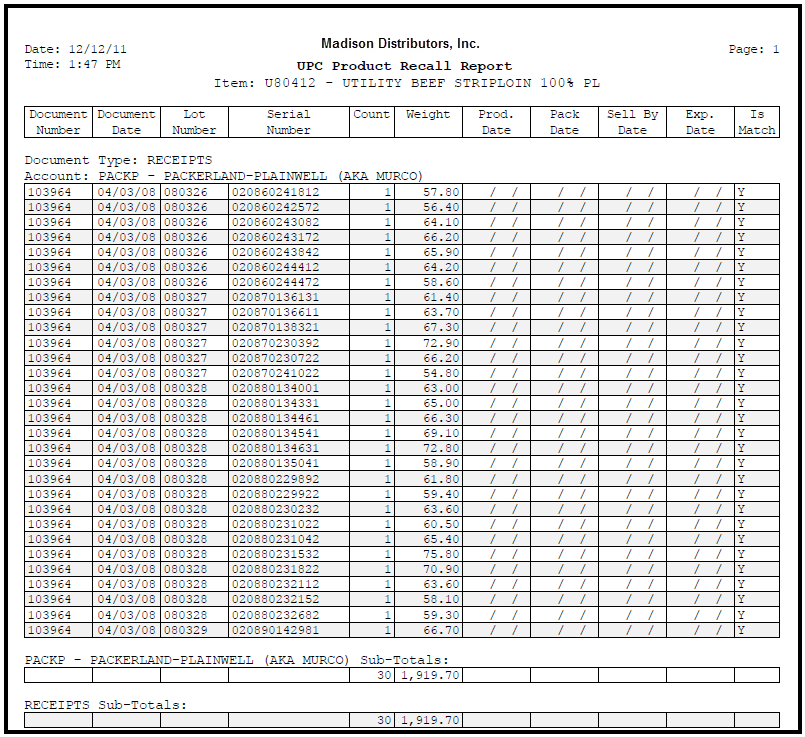
To bring this ability together, whenever a product recall is issued by the FDA, you can quickly determine what customers were provided inventory from the recalled lot's via the Product Recall Report.
In the event of a product recall this report will allow you to quickly identify those products and take action to protect the public as required by the Food Safety Modernization Act (FSMA).
entrée V3
To access the Product Recall Report use menu path: Reports > Miscellaneous > entrée UPC > Product Recall Report.
entrée V4 SQL Go to the Add-Ons ribbon menu and click the entrée.UPC option drop down menu to access the Reports menu and select Product Recall Report. |
|
How to Run the Product Recall Report
entrée V3 and entrée V4 SQL The options for this report are the same in both versions of the entrée system.
1.Select the desired Dates option (see Options below) and enter the "Data from;" and "to:" date values.
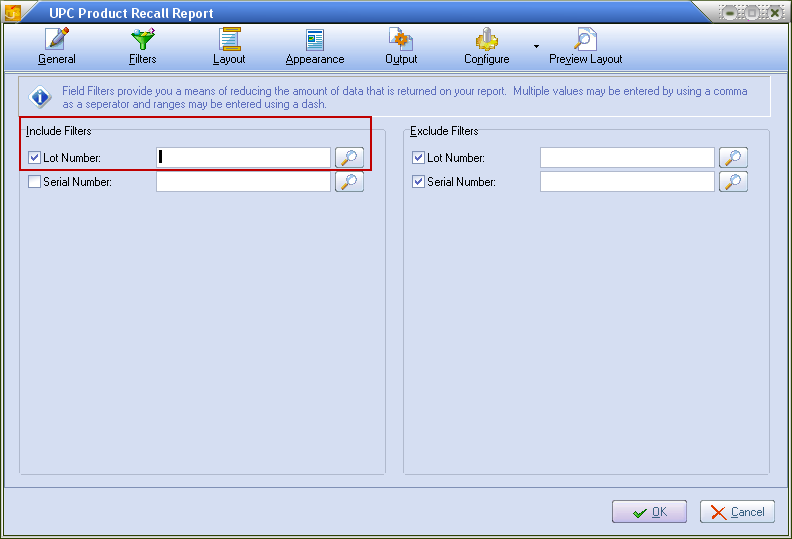
2. Click the Filters icon to access the Lot Number and Serial Number search filter options. Enter the appropriate number for the item being recalled here. Click OK.
3.Click the Layout icon. Here you will select the fields you want to see in your report. The default fields are in Data Row #1 when the screen opens. Use the Insert and Delete Field buttons to create the layout of your report.
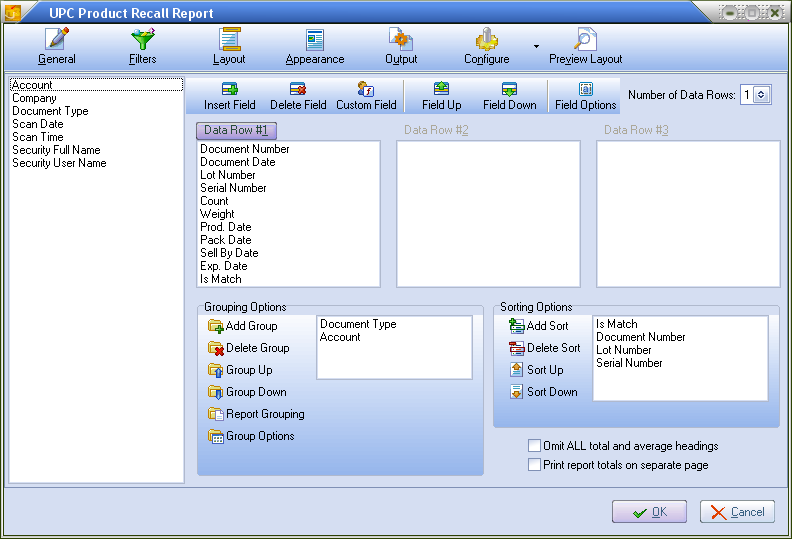
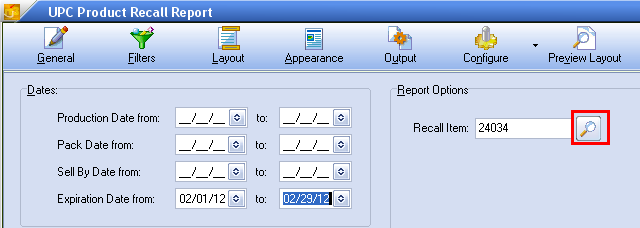
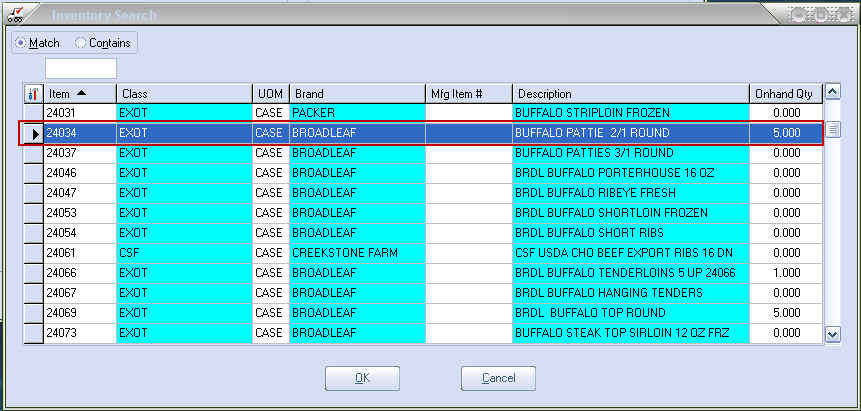
4.For Report Options enter the entrée system item # in the Recall Item text box or use the magnifying glass to open the Inventory Search dialog to find the item (see image below).
Options:
•Dates section provides four date values to be used to target the specific items being recalled. Dates include:
a.Production Date
b.Pack Date
c.Sell By Date
d.Expiration Date
•Report Options section lets you enter an item number, lot number and serial number, or search for and select the item from your inventory file.
UPC Product Recall Report Example